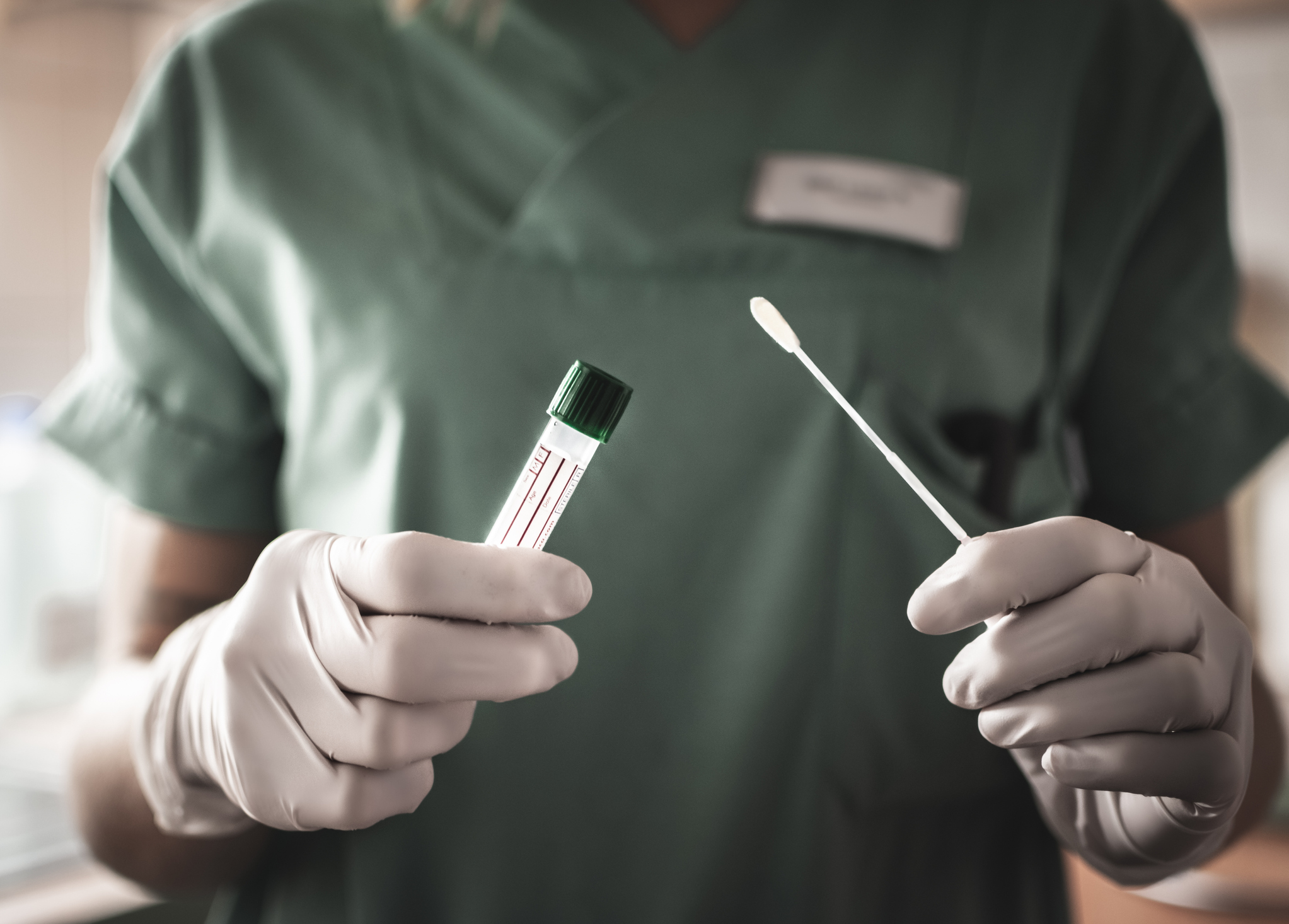
The Food and Drug Administration granted emergency clearance to the first in-home test for the coronavirus. The test is a nasal swab kit sold by LabCorp.
According to the FDA, LabCorp had submitted data showing the home test is as safe and accurate as a sample collection at a doctor’s office, hospital or other testing site.
Patients will swab their own nose using a testing kit sent by the company, and will mail it in an insulated package back to the company.
LabCorp will first make the tests available to health care workers and emergency workers who may have been exposed to the virus or have symptoms, and the company will be making the self-collection kits available to consumers in the coming weeks.
“With this action, there is now a convenient and reliable option for patient sample collection from the comfort and safety of their home,” said Dr. Stephen M. Hahn, the F.D.A. commissioner.
The company said the test will cost $119. Consumers will have to pay out of pocket for the test, a company spokesman said, and ask their insurer for reimbursement.
Medical experts said the tests could increase convenience for consumers and reduce the need for people to go to medical offices where they might expose health providers and other patients to the infection. Experts also mentioned, however, that the nasal swabs can be less accurate for testing than the gold standard specimen collection for the coronavirus that health providers perform.